Hemoglobin in colorectal cancer (DOI: 10.13140/RG.2.2.32515.99363, ISSN 2753-8176 (online))
Hemoglobin in colorectal cancer
Ana Pedro (1)
1. Gwyntwr1386 Pharmacy, Regus Chester Business Park, Heronsway,Chester CH4 9QR, United Kingdom
anapedrolaboratories@gmail.com
Colorectal cancer (CRC), also known as bowel cancer, colon cancer, or rectal cancer, is the development of cancer from the colon or rectum (parts of the large intestine)(1)
Staging of the cancer is based on both radiological and pathological findings. As with most other forms of cancer, tumor staging is based on the TNM system which considers how much the initial tumor has spread and the presence of metastases in lymph nodes and more distant organs (2).
While 30% at the patients will actually benefit from adjuvant treatment, 50% of them being already cured by the surgery and 20% of them will experience disease recurrence despite the adjuvant treatment (3) . Also, anemia is the only finding that correlates with highly advanced CRC, in the pre-operative stage (4).
Tumor cells are found to secrete hemoglobin subunits (5) what might contribute to the pathological advance of CRC.
Here, we analyze by proteomics the secretion of different hemoglobin by different colorectal cancer cell lines either overexpressing P53 or knockout for P53 (6) or treated with vitamin D (7) and from different stages of tumor progression (8-10) and benign tissues.
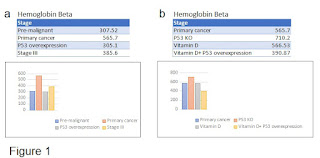
We found out that pre-malignant tissues such as human colon epithelial cells or MCF-10A breast cells express different hemoglobin subunits (Table 1). In particular, hemoglobin beta which possibly is involved in tumor progression (5) compared with pre-malignant colon tissue presents a raised score in primary tumor cells lines. Curiously, when P53 is overexpressed the score goes down again. The score is bigger again at stage III cell line, SW620, but smaller then in the primary cancer cell lines (Fig. 1a). We can see moreover, hemoglobin beta scores raises when comparing the primary tumor with the p53 knock-out cell line (Fig.1b). By adding vitamin D or by adding vitamin D and P53 overexpression the scores for hemoglobin beta go down again (Fig.1b).
The epsilon hemoglobin subunit gene is usually expressed in the embryonic yolk sac. Two epsilon chains together with two zeta chains (an alpha-like globin) constitute the embryonic hemoglobin Hb Gower I and two epsilon chains together with two alpha chains form the embryonic Gower II. Both of these embryonic hemoglobins are normally replaced by fetal hemoglobin, and then later, adult hemoglobin (11). We found out that hemoglobin epsilon is only expressed by the primary tumor and by the P53 knock-out cell line where there is an huge increase in hemoglobin epsilon score compared with the primary tumor (Figs. 2 a-b).
The gamma hemoglobin genes are usually expressed in the fetal liver, spleen and bone marrow. Two gamma chains together with two alpha chains constitute fetal hemoglobin which is normally replaced by adult hemoglobin at birth. In some beta-thalassemias and related conditions, gamma chain production continues into adulthood. The former is predominant at birth (12). Hemoglobin subunit gamma is expressed in the pre-malignant colon tissue and in the primary cancer cell lines which show an increased score in comparison to the pre-malignant tissues (Fig.3a). Moreover, the scores for this hemoglobin are slightly raised in p53 knockout and decrease with adding vitamin D (Fig.3b).
The human alpha globin gene cluster is located on chromosome 16. The alpha-2 and alpha-1 coding sequences are identical. Two alpha chains plus two beta chains constitute HbA, which in normal adult life comprises about 97% of the total hemoglobin; alpha chains combine with delta chains to constitute HbA-2, which with fetal hemoglobin makes up the remaining 3% of adult hemoglobin. Alpha thalassemias may result from deletions of both alpha globin genes; some nondeletion alpha thalassemias have also been reported (13). Hemoglobin subunit alpha is expressed by pre-malignant tissues and its score raises in the primary cancer tissues (fig.4a). P53 overexpression and in stage III tissues there are slight decreases in this protein score. This protein scores raises in the p53 knockout in comparison to the primary cancer (fig.4b), however adding vitamin D or vitaminD+p53 overexpression do slightly increase the scores for this protein.
In conclusion, all hemoglobin subunits (gamma 2, beta and alpha) are expressed in pre-malignant colon tissues except hemoglobin subunit epsilon. All hemoglobins scores are raised and hemoglobin epsilon gets expressed in the primary cancer. P53 overexpression causes a decrease in the scores of hemoglobin beta and hemoglobin alpha. In stage III there are decreases in the scores of hemoglobin beta and hemoglobin alpha. P53 knockout causes increases in all proteins scores. Adding vitamin D or vitaminD+P53 overexpression seem to provoke unclear effects in these hemoglobolin subunits scores.
Pre-malignant tissues may express hemoglobin subunits to levels which do not cause harm. However, raised expression or appearance of these proteins in the primary tumors may prompt survival and proliferation of tumor cells (5). However, overexpression of P53 may hinder cancer cell proliferation. In stage III there are decreases in the scores of hemoglobin beta and hemoglobin alpha, perhaps this explains the efficacy of adjuvant chemotherapy at this stage (14). Finally vitamin D does not seem to play any role in colorectal cancer.
Methods
Proteomic analysis was performed at the Rockefeller University, Proteomics Center as described in Hamidi et al., 2017 (15). Only proteins with Mascot scores of approximately 90 or >90 were considered (16).
The proteomic analysis excel files and each correspondent sample details were generously and kindly shared and donated by Dr. David Lyden, Weill Cornell Medical College, New York, USA. Original data files can be found at https://zenodo.org/record/7422331#.Y5THeXbP3IU
References
1. "Colon Cancer Treatment (PDQ®)". NCI. May 12, 2014.
2.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N (March 2010). "Colorectal cancer". Lancet. 375 (9719): 1030–1047.
3. Taieb and Gallois (2020). Adjuvant Chemotherapy for Stage III Colon Cancer. Cancers (Basel), 12(9): 2679.
4. Gvirtzman, et al (2021). Anemia can predict the prognosis of colorectal cancer in the pre-operative stage: a retrospective analysis. World Journal of Surgical Oncology 19, Article number: 341
5. Zheng et al (2017). Expression of β-globin by cancer cells promotes cell survival during blood-borne dissemination. Nature Communications 8, Article number: 14344
6.Liebl and Hofmann (2021). The Role of p53 Signaling in Colorectal Cancer. Cancers (Basel).May; 13(9): 2125
7. Boughanem et al. Vitamin D Intake and the Risk of Colorectal Cancer: An Updated Meta-Analysis and Systematic Review of Case-Control and Prospective Cohort Studies. Cancers (Basel). 2021 Jun;
13(11): 2814.
8. https://www.atcc.org/products/ccl-227#:~:text=SW620%20%5BSW%2D620%5D%20cells,for%20cancer%20and%20toxicology%20research.&text=Discounts%20may%20be%20available%20for%20our%20fellow%20nonprofit%20organizations.
9.https://www.atcc.org/products/ccl-247
10.https://www.atcc.org/products/htb-38#:~:text=HT%2D29%20is%20a%20cell,in%20cancer%20and%20toxicology%20research.
11. "Entrez Gene: HBE1 hemoglobin, epsilon 1".
12. "Entrez Gene: HBG2 hemoglobin, gamma G".
13. https://www.ncbi.nlm.nih.gov/gene/3039
14. Taieb and Gallois (2020). Adjuvant Chemotherapy for Stage III Colon Cancer. Cancers (Basel). 2020 Sep; 12(9): 2679.





Comments
Post a Comment